Composition and Hydration Mechanisms of Cement

Last updated on February 7th, 2022
What is cement made of?
Ordinary Portland cement chemically consists of four vital oxides. These include calcium oxide, silicon dioxide, aluminum oxide, and iron oxide. In addition, it has various minor oxides such as sulfate. However, the chemical composition of cement is of a major importance. Specifically, it affects the strength development, workability properties, and durability properties of a mixture. In fact, Portland cement gains its strength through chemical reactions between the cement powder and water (the cement hydration). However, we can better apprehend the hydration process and the reactions during this process by understanding first what is cement made of.
The chemical composition of cement
The typical chemical composition range of oxides in ordinary Portland cement in percentage by weight is shown in the following table.
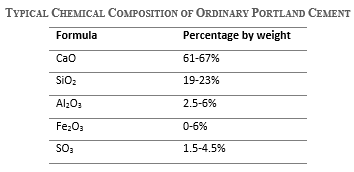
In fact, the source of these oxides can be related to the raw materials used in the manufacturing process of cement. For example, limestone is the main source of calcium oxides, and clay is the main source of both silicon dioxide and aluminum oxides, having iron oxides as impurities. In addition, gypsum is responsible for the sulfate content and not the basic clinker itself.
The chemical composition of clinker
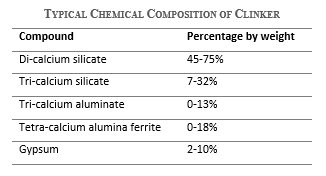
Besides, the chemical composition of clinker directly affects the chemical composition of cement, and sets the properties of the final product, which in turn determines the type of cement. Clinker consists of four major compounds, which are products of the chemical reactions between oxides during clinkering. These include di-calcium silicate C2S, tri-calcium silicate C3S, tri-calcium aluminate C3A, and tetra-calcium aluminoferrite C4AF. The typical chemical composition range of compounds in ordinary Portland cement by weight is shown in the following table.
Hydration of cement
During the hydration of cement, it reacts with water in a series of chemical reactions, mostly exothermic, to form the binding material.
The instant phenomenon
The first phenomenon occurs almost instantly when water is added. Specifically, some of the clinker sulfates and gypsum dissolve to form a sulfate alkaline solution.
The reaction of tri-calcium aluminate C3A
Afterwards, a series of reactions occur, starting with the most reactive mineral, tri-calcium aluminate. During the hydration of cement, C3A first reacts with water to form an aluminate-rich gel. This gel then reacts with sulfates to form ettringite crystals. The reaction of tri-calcium aluminate generates high heat of hydration, but only for a short period of time. Then the heat relatively decreases for several hours in the induction period.
Equation 1: The Reaction of Tri-Calcium Aluminate
C3A + 3CSH2 + 26H = C6AS3H32
The reaction of tri-calcium silicate C3S
Following the induction period of the previous reaction, tri-calcium silicate starts reacting to produce calcium silicate hydrate gel and calcium hydroxide.
Equation 2: The Reaction of Tri-Calcium Silicate
2C3S + 6H = C3S2H3 + 3CH
The reaction of unstable ettringite
Once gypsum is depleted due to the first reaction, the ettringite becomes unstable. In fact, it starts reacting with the remaining tri-calcium aluminate C3A during the hydration of cement. This reaction produces mono-sulfate aluminate hydrate crystals.
Equation 3: The Reaction of Unstable Ettringite
2C3A + 3C6AS3H32 +22H = 3C4ASH18
The reaction of di-calcium silicate C2S
Similar to the reaction of tri-calcium silicate, di-calcium silicate reacts with water to form calcium silicate hydrate gel and calcium hydroxide.
Equation 4: The Reaction of Di-Calcium Silicate
C2S + 4H = C3S2H3 + CH
The reactions of tetra-calcium aluminoferrite C4AF
In addition, tetra-calcium aluminoferrite undertakes two subsequent reactions with gypsum during the hydration of cement. First, ferrite reacts with gypsum to form alumina or ferric hydroxides, ettringite, and lime.
Equation 5: The Reaction of Tetra-Calcium Aluminoferrite with Gypsum
C4AF + 3CSH2 + 3H = C6(A or F)S3H32 + (A or F)H3 + CH
Afterwards, ferrite also reacts with the ettringite and lime produced previously, in the presence of water, to produce complex compounds. However, these compounds only fill voids and do not contribute to strength development of concrete.
Equation 6: The Reaction of Tetra-Calcium Aluminoferrite with Ettringite
C4AF + C6(A or F)S3H32 + 2CH + 23H = 3C4(A or F)SH18 + (A or F)H3
The pozzolanic reaction
Furthermore, calcium hydroxide produced by the reaction of di-calcium and tri-calcium silicates reacts with amorphous silica to produce additional calcium-silicate-hydrate gel. This reaction occurs in the presence of pozzolanic materials (natural SCMs and artificial SCMs), which are rich in amorphous silica, thus known as the pozzolanic reaction.
Equation 7: The Pozzolanic Reaction
Ca(OH)2 + H4SiO4 = CaH2SiO42H2O
Conclusion
Cement is basically made of limestone and clay. And, it chemically consists of four main oxides, including calcium oxide, silicon dioxide, aluminum oxide, and iron oxide. Mainly, the chemical composition of cement makes it a reactive material that reacts with water through a series of chemical reactions.