Characteristics of the Reactant Compounds & Hydration Products of Cement

Last updated on February 7th, 2022
The characteristics of the major cement compounds
The major compounds that react during the hydration of cement are di-calcium silicate C2S, tri-calcium silicate C3S, tri-calcium aluminate C3A, tetra-calcium aluminoferrite C4AF, and gypsum. The amounts of these compounds and how they react affect the initial and final properties of the mixture during and after the hydration process.
Tri-Calcium Aluminate C3A
Although the amount of tri-calcium aluminate C3A in cement is relatively small, it has a major effect during the hydration mechanisms. The reaction between this compound and water is very intense and highly exothermic. And in fact, it produces hexagonal crystallized calcium aluminate hydrate. This reaction can cause rapid setting in a mixture, or flash setting, if kept uncontrolled. Thereby, a set controlling agent is used, gypsum, which is added to clinker during the final manufacturing process of cement.
Hence, tri-calcium aluminate C3A is not desirable at high quantities since its reaction is highly exothermic. Also, it interacts with water or soils containing sulfates, thus affecting durability.
Gypsum
The main role of gypsum is to delay and control the intense reaction of tri-calcium aluminate C3A with water. Thus, gypsum reacts with tri-calcium aluminate C3A, forming an insoluble calcium-sulfo-aluminate compound, ettringite, around the tri-calcium aluminate particles. This formation delays and controls the reaction of tri-calcium aluminate. In fact, this phenomenon not only affects the workability of a mixture by controlling its setting time, but also gives enough time for the tri-calcium silicate C3S reaction to initiate. Then, the reaction between gypsum and tri-calcium aluminate C3A continues until one of them is depleted. During this time, the main reaction of tri-calcium aluminate is still in progress.
However, the amount of gypsum is crucial during the hydration process. If tri-calcium aluminate C3A is depleted before gypsum, the excess amount of gypsum expands causing cracks and reduced durability. Whereas, if gypsum is depleted before tri-calcium aluminate C3A, the surplus amount will eventually react with the unstable ettringite to form mono-sulfate aluminate hydrate crystals – which negatively affects the durability of concrete subjected to sulfate attacks.
Tri Calcium Silicate C3S
The tri-calcium silicate C3S reacts to produce calcium-silicate-hydrate gel (C-S-H gel), calcium hydroxide, and heat. This reaction is moderately exothermal, having a rapid rate, and is mainly responsible for the early-age strength of concrete. The hydration of tri-calcium silicate C3S affects the strength of the hardened concrete for about a year.
Di-calcium silicate C2S
Similarly, di-calcium silicate C2S reacts to produce calcium-silicate-hydrate gel (C-S-H gel), calcium hydroxide, and heat. However, this reaction produces less amounts of calcium hydroxide compared to that of tri-calcium silicate C3S . In addition, this reaction has a much slower rate, generates less heat, and is mainly responsible for the long-term strength.
Tetra-calcium aluminoferrite C4AF
The reaction of tetra-calcium aluminoferrite C4AF is somehow similar, but slower, than that of tri-calcium aluminate C3A. This compound also helps in accelerating the reaction of silicates. Its reaction is moderately exothermal, having a very rapid rate, and helps in resisting chemical attacks.
Summary of the characteristics of the main cement compounds
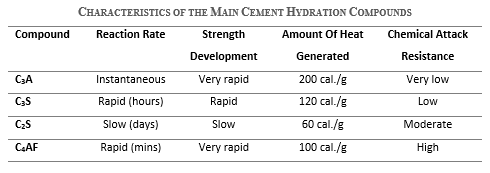
As a summary of the characteristics of the main compounds in cement hydration:
- C3A has an instantaneous reaction, very rapid strength development, high heat generation, and very low resistance to chemical attacks.
- C3S has a rapid reaction rate and strength development, moderate heat generation, and low resistance to chemical attacks.
- C2S has a slow reaction and strength development, low heat generation, and moderate resistance to chemical attacks.
- C4AF has a rapid reaction, very rapid strength development, moderate heat generation, and high resistance to chemical attacks.
The characteristics of the major hydration products of cement compounds
Hydration products are the new components produced, in their solid forms, during and after the hydration of cement. The main hydration products of cement include calcium-silicate-hydrate gel, calcium hydroxide, and ettringite.
Calcium-silicate-hydrate
Calcium-silicate-hydrate is the most important hydration component, and is the main contributor to strength. The reactions of di-calcium and tri-calcium silicates (C2S and C3S), in addition to the pozzolanic reaction, produce this compound, which forms about 50% to 60% of the cement paste. However, scientists usually refer to this component as a gel and not a crystalline material. In fact, it has no uniform structure when observed by x-ray diffraction. During hydration, C-S-H gel starts to form a coating around the tri-calcium silicate C3S grains. Specifically, C-S-H gel diffuses spines outwards in order to form the coating. Afterwards, as hydration continues, the thickness of the coating increases, pushing the spines of the adjacent particles to interlock firmly. Hence, the result is a short networked fiber structure of C-S-H with solid bonds, which greatly contributes to strength.
Calcium hydroxide
Calcium hydroxide, also known as Portlandite, is abbreviated as CH. The reactions of C2S and C3S produce this compound, which forms about 20 to 25% of the cement paste. In fact, calcium hydroxide grows in the pores or voids and around hydrated cement grains. Also, it is usually a colorless crystal, however it turns milky when subjected to calcium dioxide from air, forming calcium carbonate. This formation of calcium carbonate is the carbonation process.
Ettringite
During the reaction of tri-calcium aluminate and gypsum, sulfate ions and aluminates react to form ettringite. It is in the form of rod-like crystals in the early hydration process, or as growths in pores and cracks at later ages. Ettringite is usually stable only in the presence of gypsum.